
The CRISPR-Cas9-based gene editing technology was developed in 2020, changing the course of humanity. It is a neat system that gives us meagre humans the power to edit the code of life — the genome. Since its advent, it has been applied to various domains. Only last year, the CRISPR-edited salad hit the US markets. Soon after, the U.S. Food and Drug Administration (FDA) approved the first CRISPR-based gene therapy for sickle-cell disease.
This gene editing technology made its big news splash during the pandemic years. Emmanuelle Charpentier and Jennifer Doudna won the Nobel Prize in Chemistry for what has been called “gene technology’s sharpest tool”.
Now, plenty of people know of these gene scissors. Such breakthrough inventions often come from big universities that are constantly expanding the horizons of scientific knowledge. However, if you were to explore the history of CRISPR-Cas9, you’d see the foundation for this technology was set in a rather unlikely setting — a yoghurt industry!
Bacteriophage: The enemies of yoghurt
Danisco is a Danish company for food ingredients and other bioproducts. One of the things they make and sell are bacterial cultures for producing yoghurt from milk.
The basic science behind this is that when added to milk, certain bacteria coagulate it to make yoghurt.

Good, healthy bacteria = delicious yoghurt. Done! Well, not so fast. Enter bacteriophage (or phage, in short), a type of virus that attacks bacteria. If the virus quickly attacks the bacteria, it cannot be used to make yoghurt. So, scientists at Danisco were focused on selecting bacterial varieties (or strains) resistant to bacteriophage attacks.
How do some yoghurt bacteria resist bacteriophage?
They took a bacterial culture and exposed it to the bacteriophage. Most of the bacteria died but some survived. So, they decided to study the difference between the surviving or resistant bacteria and the non-resistant ones.
They retrieved information about the bacteria’s genomes using a technique called sequencing. After sequencing their genomes, they found that the genomes were 99+% similar, except for one section: the CRISPR array. The CRISPR array in the resistant bacteria’s genome had extra DNA sequences, or ‘spacers’. This extra element matched the DNA of the attacking virus.
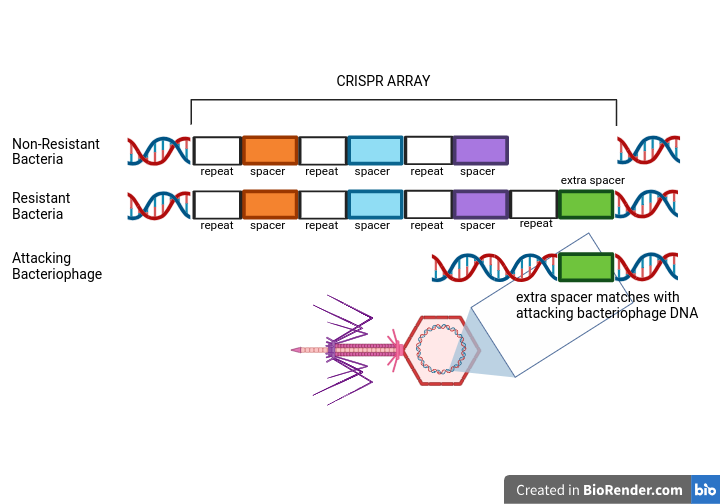
To further understand the elements involved in phage resistance, researchers at Danisco performed a set of three experiments.
Experiment 1
They exposed the bacteria to a cocktail of various bacteriophages. Upon observation, they found that the resistant bacteria incorporate additional DNA from all the attacking phages into their CRISPR region. This indicated that the acquisition of resistance may correlate with acquiring the new CRISPR spacers.
Experiment 2
To assess if the resistance acquisition depends on the spacer, they made a mutant of the same bacteria that didn’t have the spacer. Upon exposure to the bacteriophage, it failed to survive. Clearly, the resistance to attack depends on these spacers.
Experiment 3
To explore other resistance-linked elements, they investigated genes near the CRISPR genes. One such gene was Cas9, which carries information on the blueprint (sequence of amino acids in a protein) to synthesise the Cas9 protein. The researchers then created another mutant of the resistant bacteria, this time leaving the CRISPR region and the spacer intact but knocking out the cas9 gene. As a result, they observed that resistance was lost. This indicated that Cas9 is also essential for this resistance mechanism.
Later, further research revealed that the bacteria defend themselves by using the Cas9 protein as “scissors” to snip the attacking viral DNA.
The CRISPR-Cas9 gene editing system is devised
Years later, Jennifer Doudna and Emmanuelle Charpentier met at a CRISPR meeting. They chatted about science on the cobblestone streets of Puerto Rico.
At the time, little was known about how the Cas9 protein cut viral DNA and even less about how to use it to snip DNA at precise points.
So, the two decided to collaborate across continents on the “mysterious” Cas9 protein. They eventually devised a neat system using RNA to guide the DNA “scissors” to cut at precise locations. And, the rest is history!

Oftentimes, science is like building a mountain. You start with the foundation and then you build it one layer at a time. Maybe the person sitting on top of the mountain isn’t the one who laid the foundation!
Rodolphe Barrangou, former scientist at Danisco and now editor-in-chief of the CRISPR journal
Today, the CRISPR Cas9 gene editing system is being used globally across various laboratories. This landmark breakthrough is set to revolutionise medicine and health.
Isn’t it fascinating to see how scientific endeavours evolve over decades culminating in groundbreaking achievements? Like the ability to edit the code of life, which was only possible because a group of scientists just wanted to make good yoghurt!
Glossary:
Word: Genome
Sentence within the article: It is a neat system that gives us meagre humans the power to edit the code of life — the genome.
Meaning: The genome is the complete set of genetic material present in an organism, including all of its genes and DNA sequences.
Word: Cas9
Sentence within the article: One such gene was Cas9, which carries information on the blueprint (sequence of amino acids in a protein) to synthesise the Cas9 protein.
Meaning: Cas9 is a protein that acts as molecular scissors, capable of cutting DNA at specific locations, playing a crucial role in the CRISPR-Cas9 gene editing system.
Word: CRISPR-Cas9
Sentence within the article: The CRISPR-Cas9-based gene editing technology was developed in 2020, changing the course of humanity.
Meaning: CRISPR-Cas9 is a revolutionary gene editing technology that allows for precise alterations to the genome by using a guide RNA and the Cas9 protein to cut DNA at specific locations.